
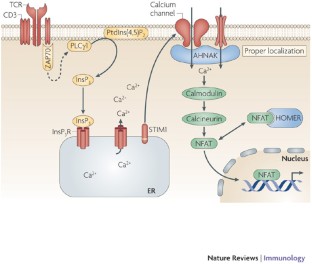
This approach revealed that VPg binds directly to the eIF4F complex, with a high affinity interaction occurring between VPg and eIF4G. To further characterize this novel mechanism of translation initiation, we have used proteomics to identify the components of the norovirus translation initiation factor complex. Noroviruses, a major cause of gastroenteritis in man, have evolved a mechanism that relies on the interaction of translation initiation factors with the virus-encoded VPg protein covalently linked to the 5′ end of the viral RNA. The proteolysis features of these proteases are described in order to provide a general guidance on the proteolytic removal of the affinity tags.read more read lessĪbstract: Viruses have evolved a variety of mechanisms to usurp the host cell translation machinery to enable translation of the viral genome in the presence of high levels of cellular mRNAs.

The most commonly used endopeptidases are enterokinase, factor Xa, thrombin, tobacco etch virus, and human rhinovirus 3C protease. So it is usually necessary to excise the tag by protease. In some cases, a large-size affinity tag, such as GST or MBP, can significantly impact on the structure and biological activity of the fusion partner protein. Here, we mainly discuss the benefits and drawbacks of several affinity or epitope tags frequently used, including hexahistidine tag, FLAG tag, Strep II tag, streptavidin-binding peptide (SBP) tag, calmodulin-binding peptide (CBP), glutathione S-transferase (GST), maltose-binding protein (MBP), S-tag, HA tag, and c-Myc tag. They were widely used to facilitate the purification and detection of proteins of interest, as well as the separation of protein complexes. Biol., 9, 933–942.Abstract: Affinity tags have become powerful tools from basic biological research to structural and functional proteomics. A., Nedospasov, S., Rosenstiel, P., Rose-John, S., and Scheller, J. Nicaise, M., Valerio-Lepiniec, M., Minard, P., and Desmadril, M. L., Velappan, N., Chasteen, L., Martinez, J. Kiss, C., Fisher, H., Pesavento, E., Dai, M., Valero, R., Ovecka, M., Nolan, R., Phipps, M. C., Pau, B., Cerutti, M., Devauchelle, G., Devaux, C., Granier, C., and Chardes, T. Monnet, C., Laune, D., Laroche-Traineau, J., Biard-Piechaczyk, M., Briant, L., Bes, C., Pugniere, M., Mani, J. M., Tramontano, A., Levitt, M., Smith-Gill, S. M., Rousseau, F., Schymkowitz, J., and Serrano, L. Towbin, H., Strahelin, T., and Gordon, J. (2001) Molecular Cloning: A Laboratory Manual, Cold Spring Harbor, Cold Spring Harbor Laboratory Press, N.
(1998) Immunology, MGU Publishers, Moscow.Įfimov, G.


 0 kommentar(er)
0 kommentar(er)
